Persistent Keratoses in Vitiligo
Felipe Cupertino, MD; 1 João Paulo Niemeyer-Corbellini, MD; 2 Tullia Cuzzi, MD, PhD; 3 Marcia Ramos-e-Silva, MD, PhD 4
ABSTRACT
Phototherapy is a well-established method for the treatment of vitiligo. It is commonly offered in the forms of sunlight, UV lamps, or lasers. 1 As ultraviolet (UV) light suppresses the cutaneous immune system and stimulates the melanocyte activity, it is a part of sunlight that can be used to treat vitiligo. 2 Purified 8-methoxypsoralen (8-MOP) with solar or artificial UV light for the treatment of vitiligo was introduced in 1948 by El-Mofty. 3 Two years later, the group led by Thomas B. Fitzpatrick determined that the artificial wavelength ultraviolet A (UVA) (320–400 nm) was the most effective one for activating 8-MOP. 4 The subsequent development of artificial sources that enabled the efficient delivery of these photons to the skin heralded the emergence of the modern psoralens and ultraviolet A (PUVA) therapy. 5
Photochemotherapy or PUVA therapy consists of a total body irradiation with UVA several times a week after taking a photosensitizer, and the most used is 8-MOP. It is taken 2 hours before irradiation, generally at a dose of 0.6 mg/kg body weight. 6 Other photosensitizers have also been used, such as 5-methoxypsoralen (5-MOP) and trimethyl-psoralen (TMP) in the form of topical agents (creams, gels, and solutions) or orally, followed by exposure to natural sunlight (the so-called PUVASOL). 7,8 For vitiligo, the irradiation is usually administered twice a week with at least 1 day, preferably 48 hours, between treatments. Only patients with extensive vitiligo are considered suitable for this kind of treatment. 9
Narrowband ultraviolet B (NB-UVB), which comprises a subset of the UVB spectrum centered at 311 nm, has gained recognition since its first application for the treatment of vitiligo by Westerhof and Nieuweboer-Krobotova in 1997. In this initial study, 67% of patients developed repigmentation following 4 months of NB-UVB therapy (given twice weekly) compared with 46% receiving psoralen-UVA. 10 This relatively robust response rate, in the setting of a better side effect profile, is the reason why NB-UVB has been considered to be the “gold standard” therapy for diffuse vitiligo. 11 Subsequent studies have validated the use of NB-UVB as monotherapy; 12-15 however, the response rates have been highly variable. 16 Recent analysis showed no significant difference in efficacy between NB-UVB and PUVA in the treatment of vitiligo. 1
PUVA therapy is associated with dose-related long-term adverse effects, the most common occurring in patients receiving the highest exposure to treatment and mainly affecting patients with types I and II of Fitzpatrick’s Phototype Classification. 6,17 The most frequent side effects reported are nausea and pruritus, which appear in approximately 15% of patients. 18 Sometimes, an intense nausea may cause drug intolerance to oral psoralen. 19 Severe side effects, such as phototoxic reaction, photoallergic reaction, and subsequent hyperkeratosis and skin cancer, have been related to PUVA therapy; therefore, careful UVA dosimetry and delivery are required. 20 Also, psoralens are contraindicated in children under 12 years old, pregnant women, and lactating women, 14 and should be indicated with extreme caution to patients with a history of skin cancer (melanoma or non-melanoma), pre-malignant skin lesions, cataracts, alteration of liver and kidney function, skin type I, concomitant immunosuppressive therapy, or associated phototoxic treatment.
Despite fewer complications in phototherapy with NB-UVB compared to UVA/PUVA, the treatment is not free of side effects. The most common are erythema, mild burning or pain, mild to moderate itching, and sensitive skin, which are well tolerated by most patients and generally disappear within hours after treatment. 1 Protective measures should be adopted in patients with a history of herpes simplex. The induction of photodegenerative changes by UVB is well established. 21 Paradoxically, skin cancer appears to occur rarely in vitiliginous skin despite the lack of protection to UV radiation by melanin. 22
Phototherapy-induced keratosis is rarely reported and was first described as PUVA keratosis (PK), a distinct entity, in patients with psoriasis under treatment with PUVA 23 and later in patients with vitiligo under the same treatment. 24
The authors describe the occurrence of keratosis in vitiligo lesions of three women patients, type IV of Fitzpatrick’s Phototype Classification, from Rio de Janeiro, Brazil, undergoing NB-UVB phototherapy for the treatment of non-segmental generalized vitiligo. The keratosis started after oral PUVASol or PUVA cabin therapy.
CASE REPORTS
Patient 1
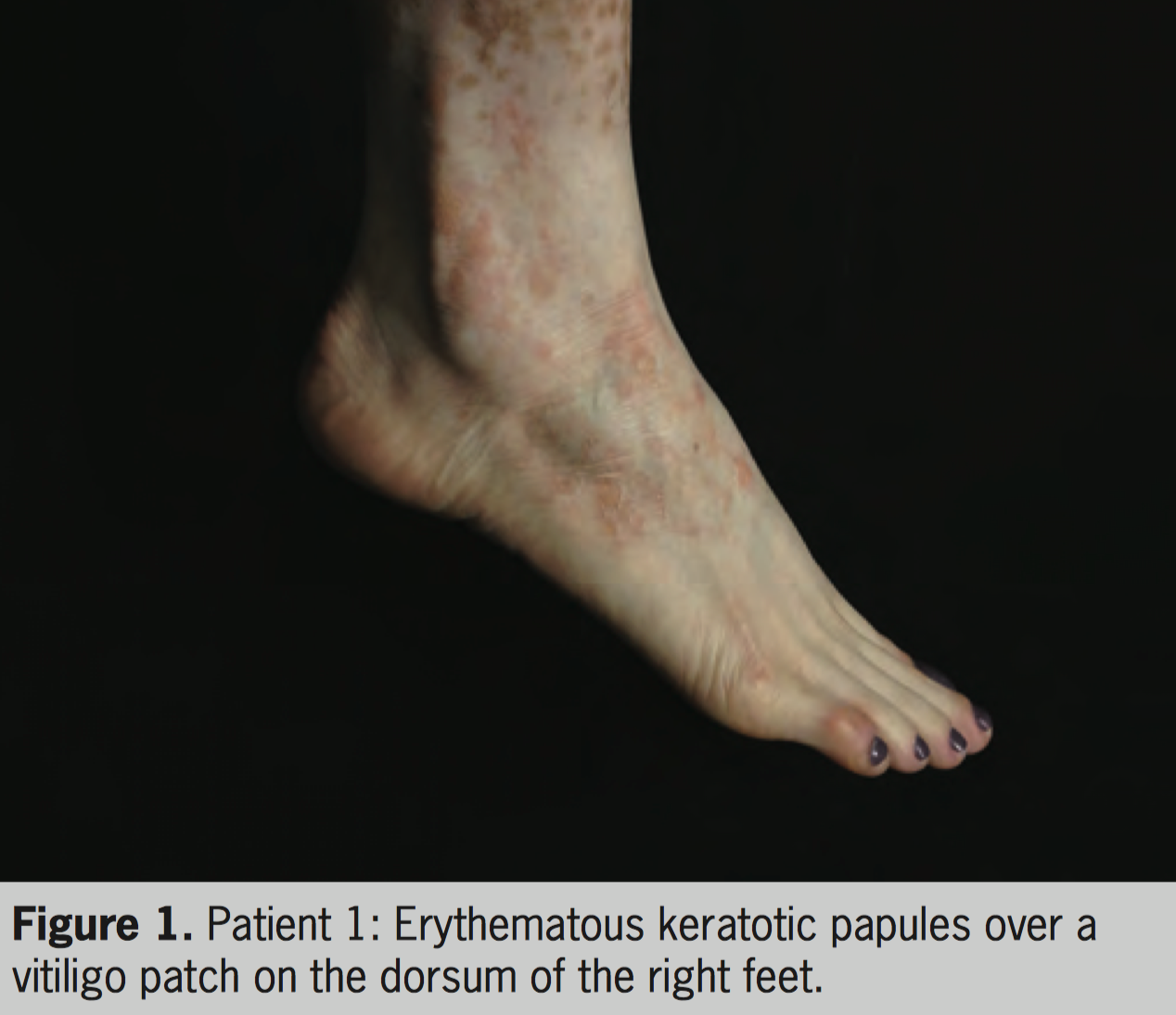
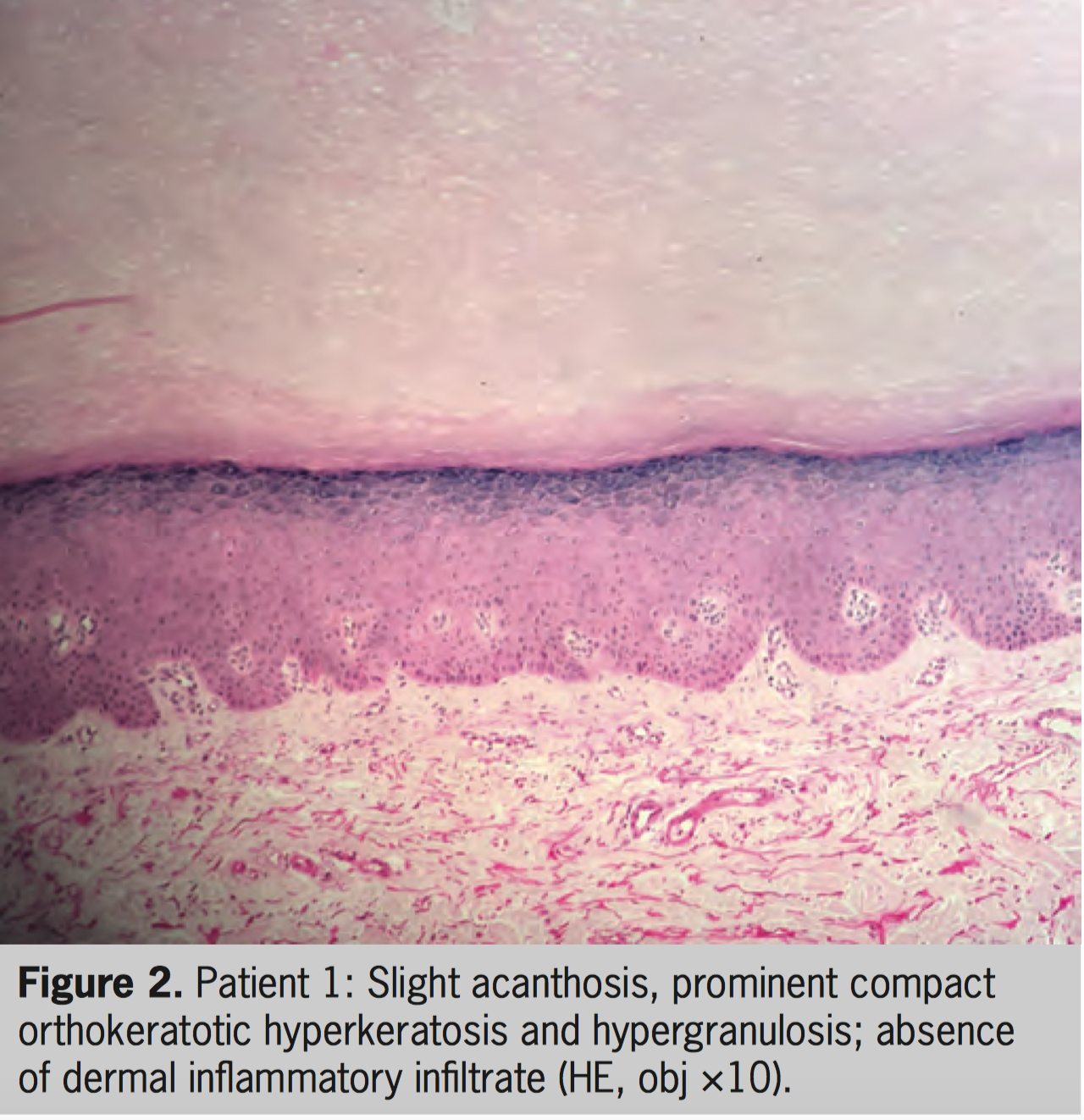
A 32-year-old woman with vitiligo started treatment with oral 8-MOP plus UVA since the age of 4. After 350 PUVA sessions (total of 1.978 J/cm 2 ), circumscribed and slightly erythematous keratotic lesions were observed on the dorsum of feet and ankles. Diclofenac 3% gel was prescribed along with close monitoring of the patient. The treatment was changed to NB-UVB and a biopsy specimen was taken, revealing compact hyperkeratosis without cytologic atypia, compatible with “reactive hyperkeratosis.” The patient missed the follow-up and returned 5 years later, with a new vitiligo spread and maintenance of keratotic erythematous lesions (Figure 1). The keratotic lesions were restricted to the lesions of vitiligo on the feet, with no other keratotic lesions elsewhere. A new biopsy showed slight acanthosis, prominent compact orthokeratotic hyperkeratosis, and hypergranulosis (Figure 2). The patient is currently under treatment with NB-UVB (total of 107 sessions) with excellent repigmentation and maintenance of keratotic lesions on her feet, which are kept closed during the treatment.
Patient 2
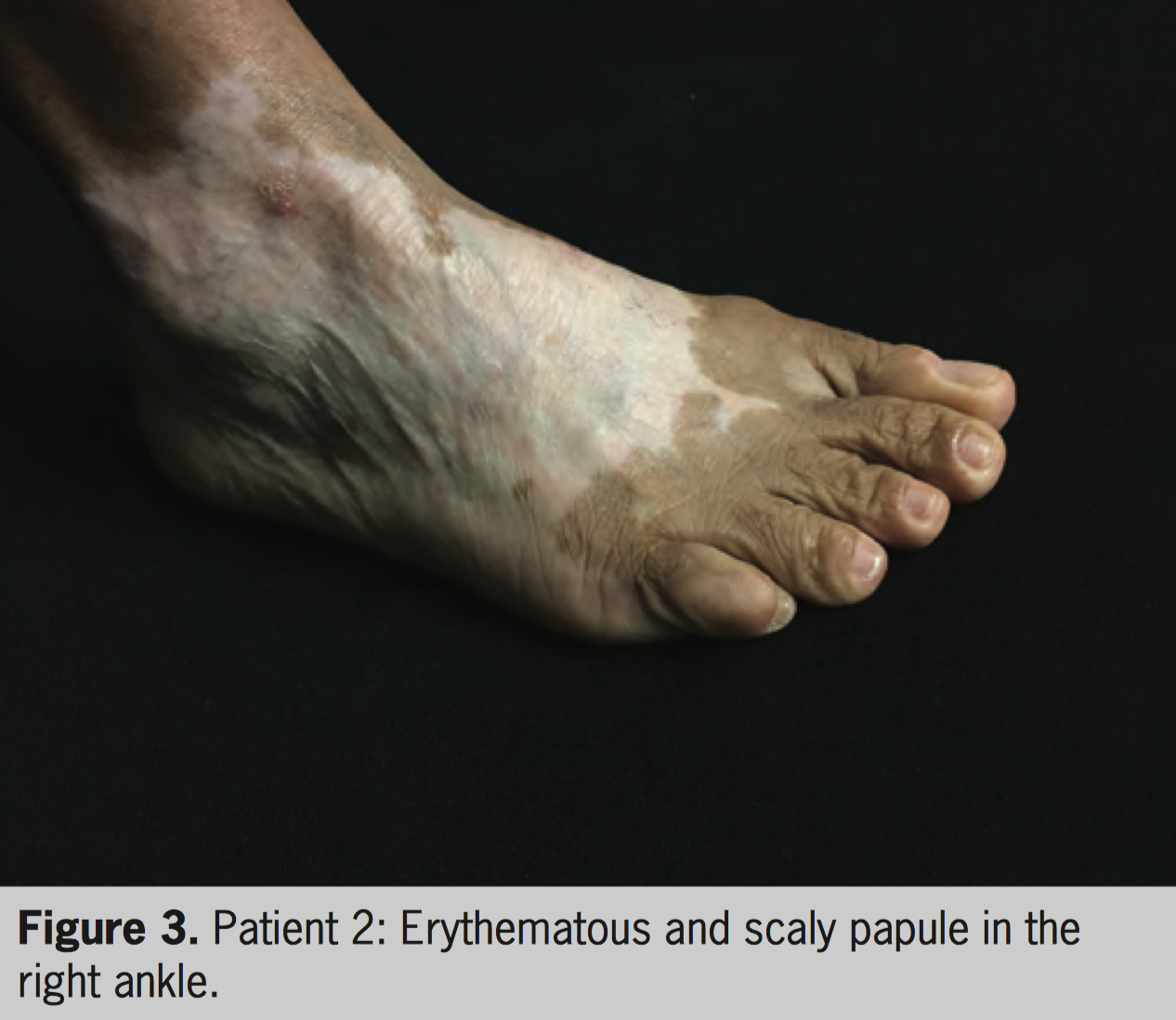
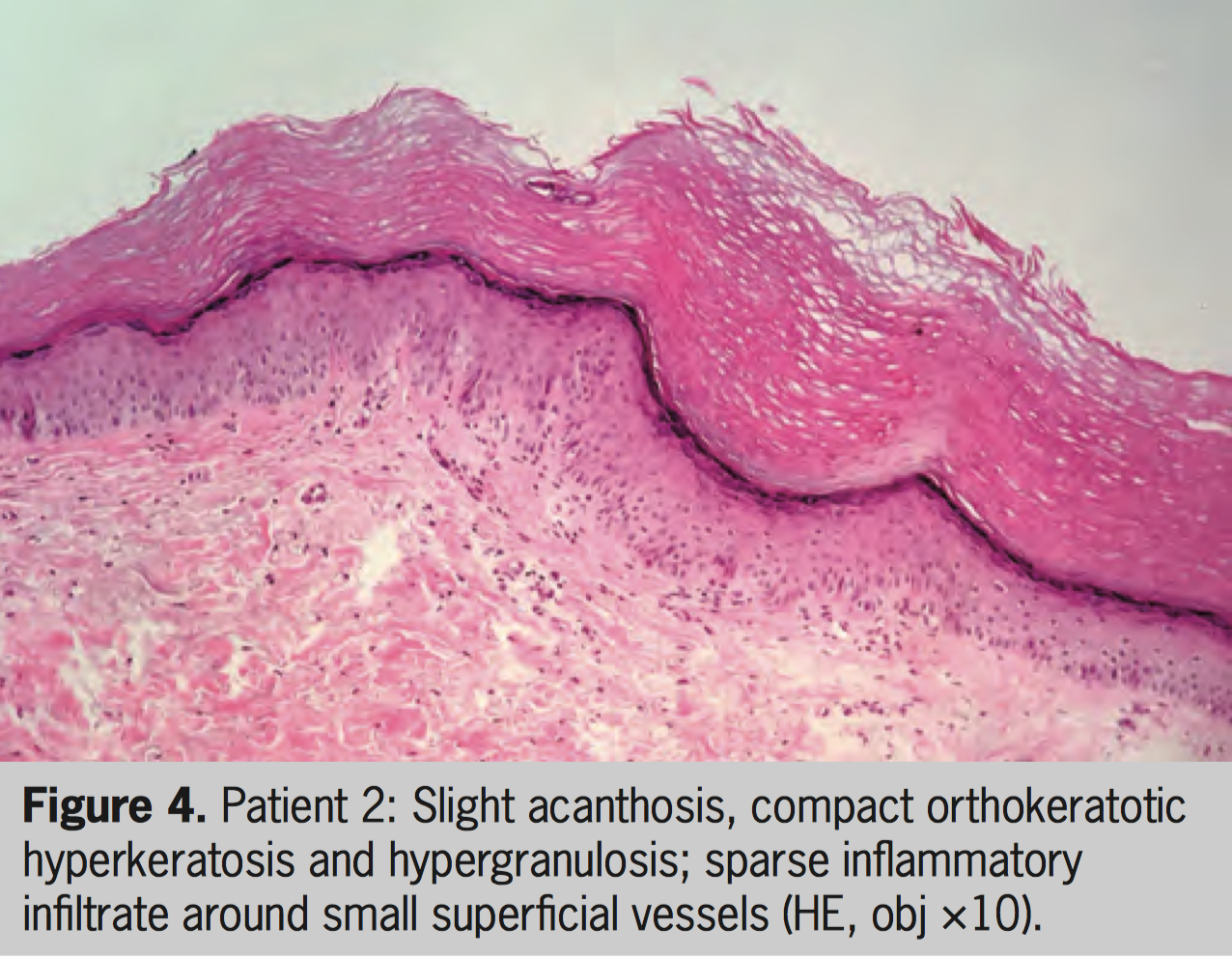
A 49-year-old woman with vitiligo diagnosed at the age of 6 started treatment with PUVA in 2009. After approximately 100 sessions (total of 328 J/cm²), she presented erythematous and keratotic lesions on the dorsum and lateral side of the feet (vitiliginous areas). Moisturizers were prescribed and chemical cauterization was performed. Close monitoring of the lesions was initiated. After a total of 233 sessions (total of 1.308 J/cm²) of PUVA, the treatment was suspended. In early 2016, with worsening of vitiligo, she restarted treatment, this time with NB-UVB, and one of the lesions (Figure 3) was biopsied. Again, the keratotic lesions were restricted to the vitiliginous skin on the feet, with no other keratotic lesions elsewhere. Histopathologic examination showed mild acanthosis, compact orthokeratotic hyperkeratosis and hypergranulosis, and the presence of a sparse inflammatory infiltrate around small superficial vessel (Figure 4). The patient continued her treatment with NB-UVB (100 sessions to date).
Patient 3
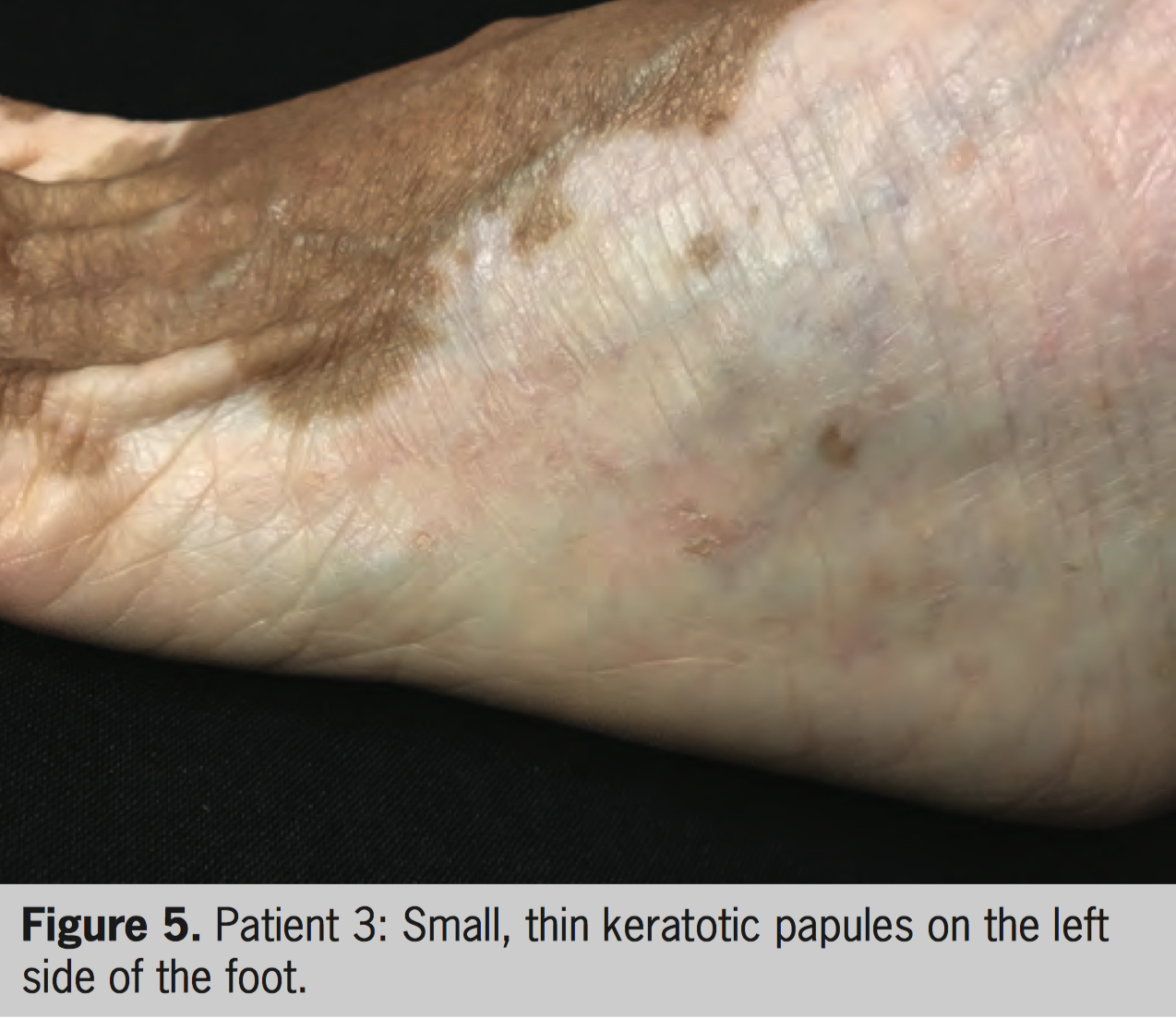
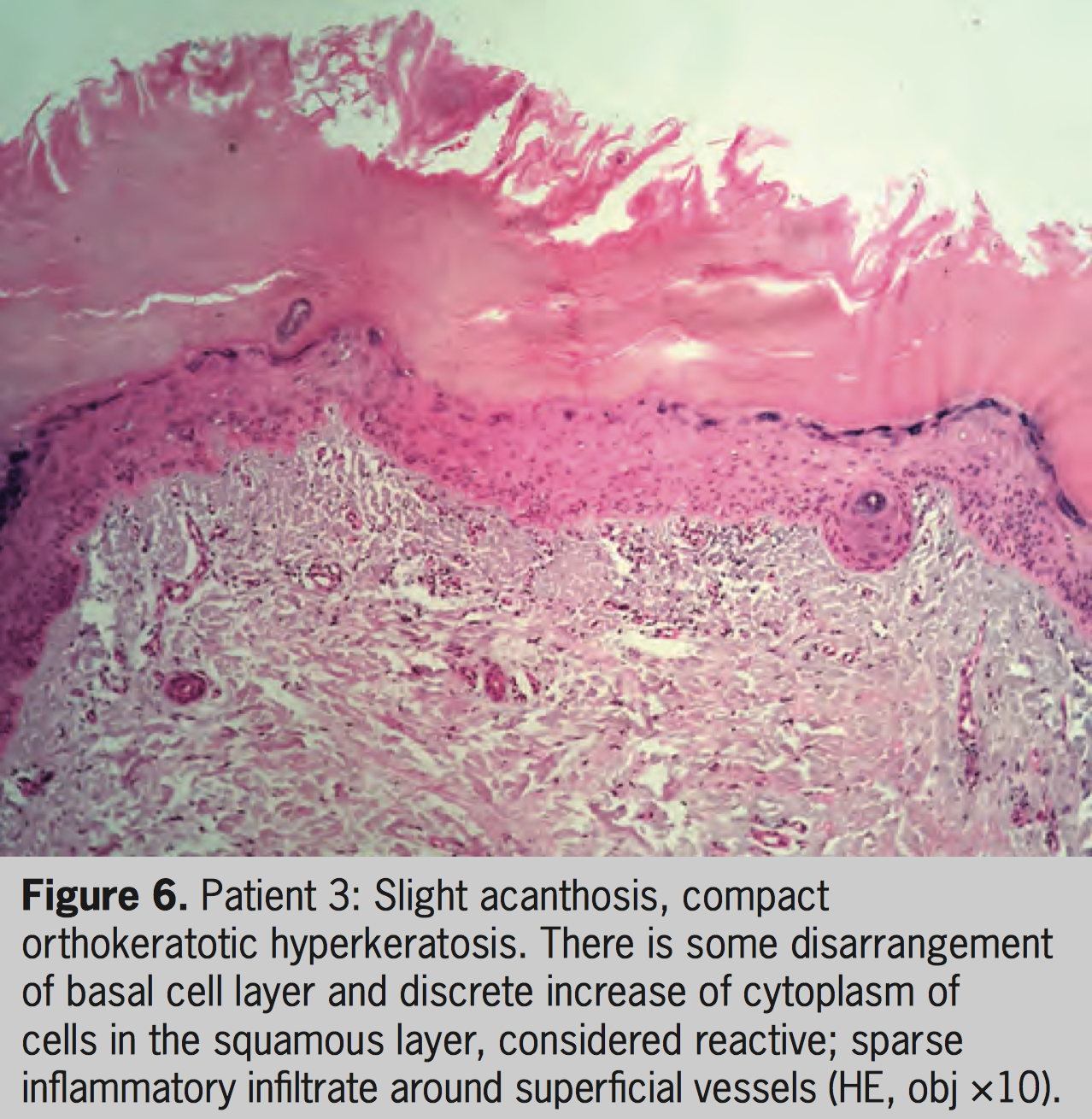
A 58-year-old woman with vitiligo for 56 years presented since 2014 round keratotic papules on achromic areas on the dorsal and lateral regions of the feet, which emerged 5 years after continuous treatment with topical psoralen and sun exposure (topical PUVASol) (Figure 5). Biopsy of one of the lesions showed histopathologic features compatible with reactive keratosis; no sign of malignancy was seen (Figure 6). The patient is now under treatment with NB-UVB (259 sessions to date), with good repigmentation of vitiligo lesions. The keratoses maintain their number and clinical pattern unchanged so far.
DISCUSSION
PK was first described in 1991 in a long-term follow-up study of patients with psoriasis. The authors defined a lesion of PK as a raised papule with a broad base and a diameter of several millimeters to about 1 cm. 23 Later, the same group described the clinical and histopathologic features of PK. They were predominantly observed in older men, primarily on non-sun-exposed skin, such as the trunk and thighs. Punch biopsy specimens revealed epidermis with a variable degree of acanthosis, orthokeratosis, focal parakeratosis, and some apoptotic cells. In half of the specimens, papillomatosis was observed. Neither viral changes nor typical features of psoriasis were observed. Although only mild atypia of keratinocytes was found in 50% of the cases, 4 of the 13 patients with PUVA keratoses developed a total of 10 lesions of nonmelanoma skin cancer (NMSC), a number significantly high as compared with the number of skin cancer in the group of psoriasis patients without PK who also had received long-term PUVA treatment. 25
In 2000, a series of 10 psoriatic patients with a distinctive pattern of keratosis involving hands and feet was reported as being the first report of a manifestation of prolonged PUVA therapy. The mean dose of whole-body PUVA therapy was 2.081 J/cm 2 and six patients had a previous history of NMSC. The keratoses were well defined, rounded, usually less than 5 mm in diameter and mostly found on the lateral or medial margins of palms and soles and also on the sides of the fingers. The overlying sharp-edged scale could often be removed, leaving a shallow depression with an erythematous base. All affected patients had more typical PUVA-induced keratoses at other sites, especially on the thighs. On histopathologic examination, parakeratosis was present in all specimens but tended to be discontinuous. There was variable hyperkeratosis. The granular cell layer was consistently diminished or absent. Some of the lesions had occasional dyskeratotic cells, although there was little or no dysplasia. 26
The occurrence of PK in 12 patients during treatment for vitiligo was described in 2006. All patients had skin phototype V, and the mean cumulative UVA dose was 2.024 J/cm 2 . Clinically, they were small (2–7 mm in diameter), well-defined round keratotic lesions covered with adherent sharp-edged scales mainly on the extensor aspects of the extremities, especially feet and hands. All lesions developed on the vitiligo skin. Histologically, PK showed marked hyperkeratosis with a variable degree of acanthosis and foci of incomplete parakeratosis in some lesions. Lesions showed no elastotic changes of collagen in the dermis, and 5 of the 12 lesions showed mild atypia of keratinocytes on the lower epidermis. As extremities are non-exposed areas of skin according to the dressing customs of these patients and there were no elastotic changes on histologic examination, the authors excluded the role of sunlight in inducing the lesions. 24
Recently, the development of lichenoid papules in vitiliginous skin with long-term systemic NB-UVB phototherapy was reported, 27 but no keratosis induced by UVB-NB treatment has been described in the literature so far.
The etiology of PK is still uncertain. They are considered as a mild irritative or proliferative response to UV in psoriatic patients. 28 As PUVA was first introduced to treat psoriasis, PUVA-related squamous cell carcinoma (SCC) has been reported in psoriasis patients. 29–31 By contrast, only three case reports described the occurrence of SCC after prolonged PUVA therapy. 32-34
The importance of stratum corneum thickening as a protective mechanism against UV has been emphasized in vitiligo lesions. 35 Vitiligo skin treated with targeted phototherapy undergoes progressive photoadaptation with a corresponding increase in the minimal erythemal dose. 36 In normal skin, the same finding may be attributable to the increase in melanogenesis and in areas affected by vitiligo, and this may be due to photoprotective mechanisms other than melanin, such as the epidermal layer thickness, optical properties, and chromophores. 37
Sun-exposed vitiligo skin does not usually show features of actinic damage. Recent studies demonstrated reduced risk of skin cancer in patients with vitiligo. One study compared the relative risk (RR) of melanoma and NMSC in a large cohort of vitiligo patients with patients seen for vascular surgery. Overall, the crude RR for melanoma was 0.24, and for NMSC, it was 0.19 in patients with vitiligo compared with those with a non-dermatological condition. 38 In another case-control study among 1,307 vitiligo patients and their healthy partners, vitiligo was associated with a threefold decreased probability of melanoma and NMSC. 39 These evidences of a negative association between vitiligo and skin cancer increase the comfort level in using longer durations of phototherapy for vitiligo, as long as permanent follow-up is performed.
This is the second case report of keratoses in patients with vitiligo to date. In the previous report, feet lesions were also common, but their local dressing habits keep the feet covered most of the time. Our patients are somewhat different, as the dorsum of the feet is an area frequently exposed to the sunlight by Brazilian women and there were no other keratotic lesions, even in vitiligo areas elsewhere in the body of these patients. Another different characteristic is that the lesions persisted even after discontinuation of PUVA treatment or change to NB-UVB with no dysplastic change. Considering the presence of lesions only in areas commonly exposed to the sunlight in our country and maintenance for long periods even without treatment with PUVA or NB-UVB, the authors believe that daily sunlight exposure may play an important role in the reported patients. As the biopsies of our three patients did not show dysplasia, we chose to maintain UVB-NB treatment due to good clinical response for vitiligo and to perform a regular follow-up of the keratotic lesions.
CONCLUSIONS
This study reports the presence of persistent keratosis on vitiligo areas in the dorsum and lateral aspects of the feet of three women phototype IV patients.
In some aspects, our patients present keratoses similar to those described by Hassab-El-Naby et al. 24 Their patients developed lesions over vitiligo areas on extensor surface of the feet; however, the keratotic lesions of our patients are found in areas commonly exposed to the sun according to our habits and do not present atypical keratinocytes on the histopathologic examination. In addition, in their patients lesions remained even after a long period of non-exposure to PUVA therapy or oral psoralens, which lead the authors to believe that sun exposure may have had a role in their maintenance.
As these keratoses are usually asymptomatic and many patients are unaware of their presence, we believe that they are underreported. Physicians must advise patients undergoing phototherapy to do a regular self-examination, especially those in treatment with home devices, and to report any new or changing lesions. In addition, dermatologists should perform a regular thorough examination in these patients, with special attention to areas exposed to the sun, in search of any suspicious lesion.
Further studies are necessary to identify the prevalence and the consequences of the presence of these lesions in patients with vitiligo.
REFERENCES
- Xiao BH, Wu Y, Sun Y, et al. Treatment of vitiligo with NB-UVB: A systematic review. J Dermatolog Treat. 2015;26:340–346.
- Schwarz T. Mechanisms of UV-induced immunosuppression. Keio J Med. 2005;54:165–171.
- El-Mofty AM. A preliminary clinical report on the treatment of leucoderma with Ammi majus linn. J Royal Egypt Med Assoc. 1948;31:651–665.
- Lerner AB, Denton CR, Fitzpatrick TB. Clinical and experimental studies with 8-methoxypsoralen in vitiligo. J Invest Dermatol. 1953;20:299–314.
- Bethea D, Fullmer B, Syed S, et al. Psoralen photobiology and photochemotherapy: 50 years of science and medicine. J Dermatol Sci. 1999;19:78–88.
- Pathak MA, Mosher DB, Fitzpatrick TB. Safety and therapeutic effectiveness of 8-methoxypsoralen, 4,5’,8-trimethylpsoralen, and psoralen in vitiligo. Natl Cancer Inst Monogr. 1984;66:165–173.
- McNeely W, Goa KL. 5-Methoxypsoralen. A review of its effects in psoriasis and vitiligo. Drugs. 1998;56:667–690.
- Grimes PE. Vitiligo. An overview of therapeutic approaches. Dermatol Clin. 1993;11:325–338.
- Kovacs SO. Vitiligo. J Am Acad Dermatol. 1998;38:647–666. 10 Westerhof W, Nieuweboer-Krobotova L. Treatment of generalized vitiligo with UV-B radiation vs topical psoralen plus UV-A.
- Arch Dermatol. 1997;133:1525–1528.
- Bacigalupi R, Postolova A, Davis R. Evidence-based, non-surgical treatments for vitiligo. Am J Clin Dermatol. 2012;13:217–237.
- Scherschun L, Kim JJ, Lim HW. Narrow-band ultraviolet B is a useful and well-tolerated treatment for vitiligo. J Am Acad Dermatol. 2001;44:999–1003.
- Natta R, Somsak T, Wisuttida T, Laor L. Narrowband ultraviolet B radiation therapy for recalcitrant vitiligo in Asians. J Am Acad Dermatol. 2003;49:473–476.
- Kanwar AJ, Dogra S, Parsad D, Kumar B. Narrow-band UVB for the treatment of vitiligo: An emerging effective and well-tolerated therapy. Int J Dermatol. 2005;44:57–60.
- Nicolaidou E, Antoniou C, Stratigos AJ, et al. Efficacy, predictors of response, and long-term follow-up in patients with vitiligo treated with narrowband UVB phototherapy. J Am Acad Dermatol. 2007;56:274–278.
- El-Mofty M, Mostafa W, Esmat S, et al. Phototherapy in vitiligo: A comparative evaluation of various therapeutic modalities. J Egypt Women Dermatol Soc. 2012;9:123–135.
- Gupta AK, Anderson TF. Psoralen photochemotherapy. J Am Acad Dermatol. 1987;17:703–734.
- Melski JW, Tanenbaum L, Parrish JA, et al. Oral methoxsalen photochemotherapy for the treatment of psoriasis: A cooperative clinical trial. J Invest Dermatol. 1977;68:328–335.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;36:117–125.
- Morison WL, Baughman RD, Day RM, et al. Consensus workshop on the toxic effects of long-term PUVA therapy. Arch Dermatol. 1998;134:595–598.
- Pacifico A, Leone G. Photo(chemo)therapy for vitiligo. Photodermatol Photoimmunol Photomed. 2011;27: 261–277.
- Schallreuter KU, Tobin DJ, Panske A. Decreased photodamage and low incidence of non-melanoma skin cancer in 136 sun-exposed Caucasian patients with vitiligo. Dermatology. 2002;204:94–201.
- Bruynzeel, Bergman W, Hartevelt HM, et al. “High single-dose” European PUVA regimen also causes an excess of non-melanoma skin cancer. Br J Dermatol. 1991;124:49–55.
- Hassab-El-Naby HM, Al-Ajmi HS, Al-Sabah HY, Kajeji MM. PUVA keratosis in vitiligo. J Eur Acad Dermatol Venereol. 2006;20:1013–1014.
- Van Praag MC, Bavinck JN, Bergman W, et al. PUVA keratosis. A clinical and histopathologic entity associated with an increased risk of nonmelanoma skin cancer. J Am Acad Dermatol. 1993;28:412–417.
- Turner RJ, Sviland L, Charlton F, Farr PM. PUVA-related punctate keratoses of the hands and feet. J Am Acad Dermatol. 2000;42:476–479.
- Aljasser M, Richer V, Ball N, et al. Photolichenoid papules within vitiligo induced by narrowband UVB phototherapy. J Eur Acad Dermatol Venereol. 2016;30:1428–1429.
- Gupta AK, Siegel MT, Noble SC, Rasmussen JE. Keratoses in patients with psoriasis: A prospective study in fifty-two inpatients. J Am Acad Dermatol. 1990;23:52–55.
- Stern RS, Lunder EJ. Risk of squamous cell carcinoma and methoxsalen (psoralen) and UV-A radiation (PUVA). A meta-analysis. Arch Dermatol. 1998;134:1582–1585.
- Hirose-Matsuda H, Okamoto O, Sakai T, et al. Multiple malignant changes and recurrent infections in the skin associated with long-term exposure to ultraviolet light and topical psoralen plus ultraviolet A therapy. J Dermatol. 2015;42:536–537.
- Maiorino A, De Simone C, Perino F, et al. Melanoma and nonmelanoma skin cancer in psoriatic patients treated with highdose phototherapy. J Dermatolog Treat. 2016;27:443–447.
- Buckley DA, Rogers S. Multiple keratosis and squamous cell carcinoma after PUVA treatment of vitiligo. Clin Exp Dermatol. 1996;21:43–45.
- Takeda H, Mitsuhashi Y, Kondo S. Multiple squamous cell carcinoma in situ in vitiligo lesions after long-term PUVA therapy. J Am Acad Dermatol. 1998;38:268–270.
- Park HS, Lee YS, Chun DK. Squamous cell carcinoma in vitiligo lesion after long-term PUVA therapy. J Eur Acad Dermatol Venereol. 2003;17:578–580.
- Gniadecka M, Wulf HC, Mortensen NN, Poulsen T. Photoprotection in vitiligo and normal skin: A quantitative assessment of the role of stratum corneum, viable epidermis and pigmentation. Acta Derm Venereol. 1996;76:429–432.
- Rivard J, Hexsel C, Owen M, et al. Photoadaptation of vitiliginous skin to targeted ultraviolet B phototherapy. Photodermatol Photoimmunol Photomed. 2007;23:258–260.
- El-Khateeb EA, Ragab NF, Mohamed SA. Epidermal photoprotection: Comparative study of narrowband ultraviolet B minimal erythema doses with and without stratum corneum stripping in normal and vitiligo skin. Clin Exp Dermatol. 2011;36:393–398.
- Paradisi A, Tabolli S, Didona B, et al. Markedly reduced incidence of melanoma and nonmelanoma skin cancer in a nonconcurrent cohort of 10,040 patients with vitiligo. J Am Acad Dermatol. 2014;71:1110–1116.
- Teulings HE, Overkamp M, Ceylan E, et al. Decreased risk of melanoma and nonmelanoma skin cancer in patients with vitiligo: A survey of 1307 patients and their partners. Br J Dermatol. 2013;168:162–171.